JZ Modifier Requirements: What You Need to Know

 New modifier JZ requirements will show Medicare if providers are using pharmaceuticals efficiently by identifying the amount of unused and discarded drugs from single-dose containers or single-use packages. One way to remember the correct use of these drugs is waste vs zero-waste. Modifier JW is used to report wasted drugs vs modifier JZ is used to report when there is zero waste.
New modifier JZ requirements will show Medicare if providers are using pharmaceuticals efficiently by identifying the amount of unused and discarded drugs from single-dose containers or single-use packages. One way to remember the correct use of these drugs is waste vs zero-waste. Modifier JW is used to report wasted drugs vs modifier JZ is used to report when there is zero waste.
JW= Waste (Drug amount discarded/not administered to any patient)
JZ= Zero Waste (Zero drug amount discarded/not administered to any patient)
A recently published CMS FAQ indicated that this new modifier was introduced due to the “observed low compliance with JW modifier use … JZ modifier will be required on claims for single-dose container drugs to attest when there are no discarded amounts.”
Critical Access Hospitals (CAHs) are required to comply with this new billing requirement.
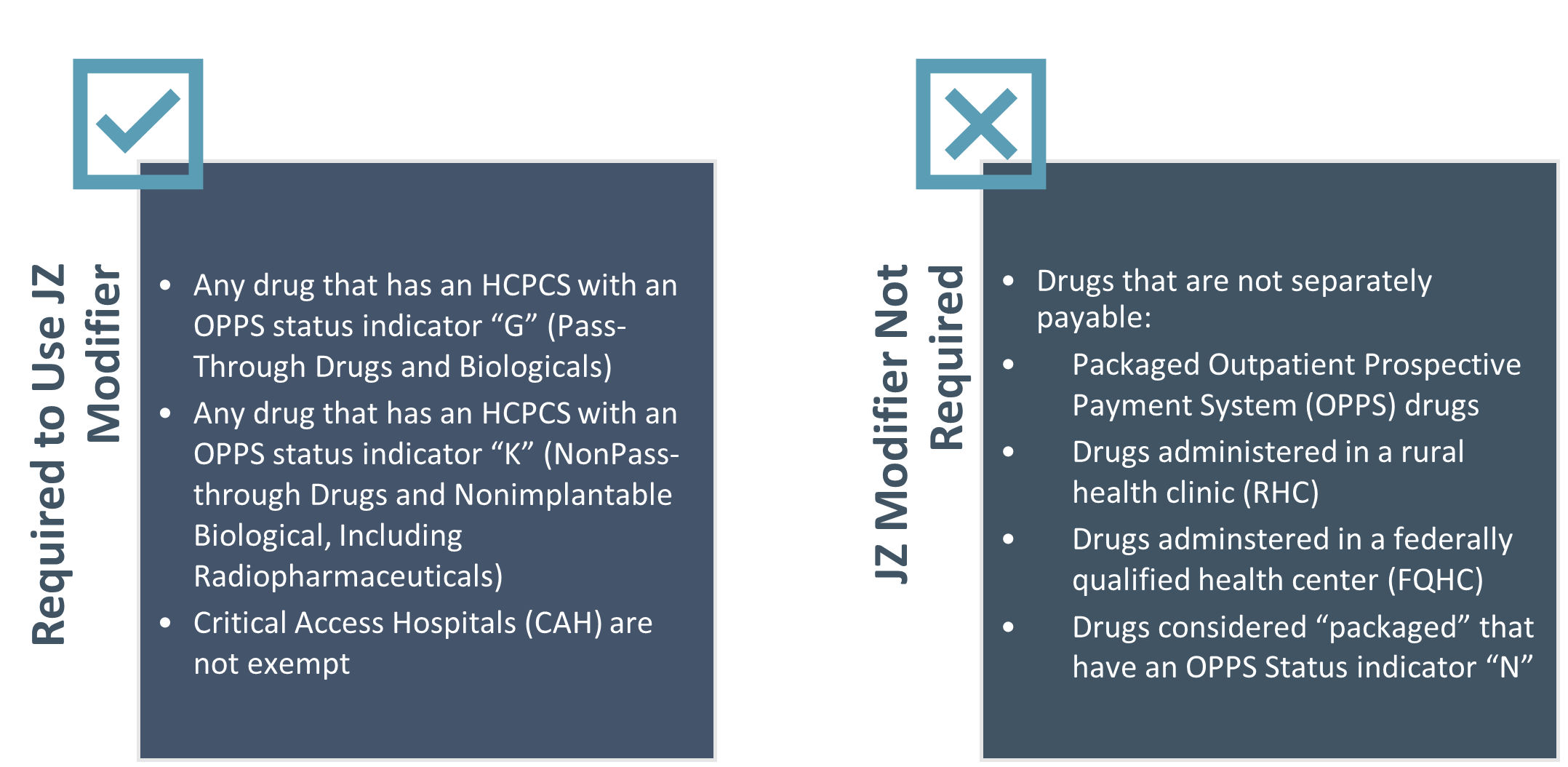
CMS clarified that modifiers JW and JZ do not apply for drugs that are not separately payable. CMS defines drugs as not separately payable as those that are packaged Outpatient Prospective Payment System (OPPS) or drugs administered in a rural health clinic (RHC) or federally qualified health center (FQHC).
Specifically, Question 19 in the FAQ states that both modifiers JW and JZ apply to any drug that has an HCPCS with an OPPS status indicator “G” (Pass-Through Drugs and Biologicals) or “K” (NonPass-through Drugs and Nonimplantable Biological, Including Radiopharmaceuticals). If the drug is considered “packaged” and has an OPPS Status indicator “N” then modifier JW or JZ are not required to be reported.
For example:
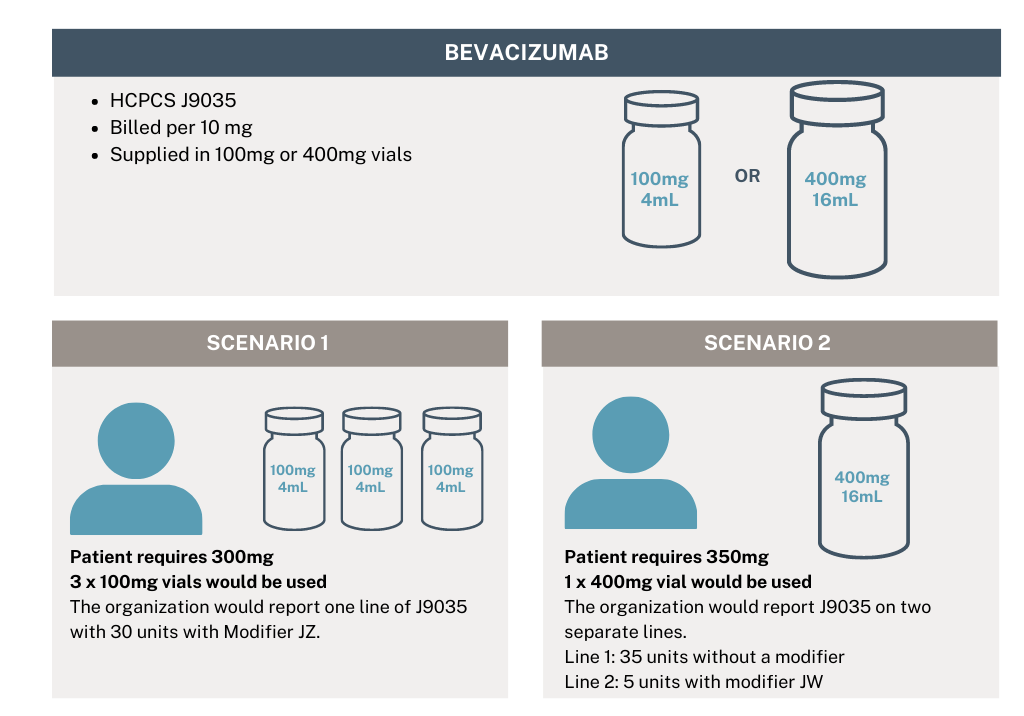
Organizations need to make sure their formularies are set up correctly now to avoid potential denials or delays in payment. All pharmaceuticals with a status indicator of either G or K will need to be submitted as indicated in the example above and have a JZ or JW modifier on the claim.
References:
Disclaimer of Liability: This publication is intended to provide general information to our clients and friends. It does not constitute accounting, tax, investment, or legal advice; nor is it intended to convey a thorough treatment of the subject matter.


